Molecules That Can Cross the Cell Membrane by Simple Diffusion Are
Cell Membrane: What types of molecules tin pass through the cell plasma membrane?
What are the factors that determine whether a molecule tin cross a cell membrane?
There are 3 important factors that determine whether a molecule tin move or cross through a cell membrane: 1) Molecular Size, 2) Concentration, and iii) Molecular Charge or Polarity.
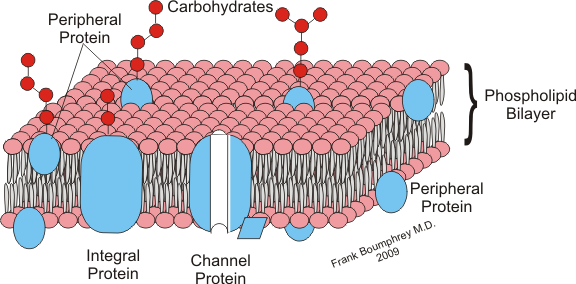
1. Molecular Size
The larger the molecule is, the harder information technology is to cross through the cell membrane.
The smaller the molecule is, the easier it is to cross through the cell membrane.
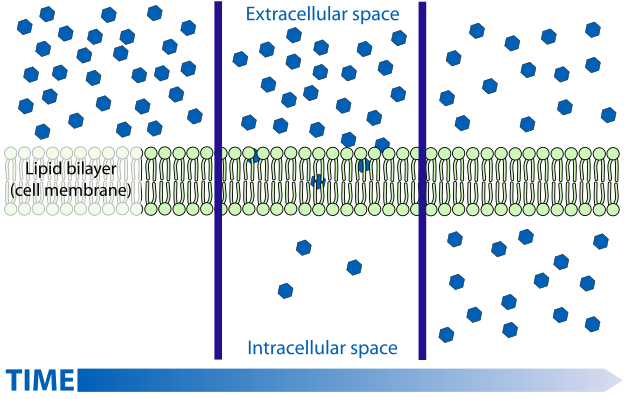
ii. Concentration
Molecules similar spaces that are less crowded, so when one side of the prison cell membrane has a low concentration of that aforementioned type of molecule, the molecules can cantankerous the cell membrane more easily. For example, when there is a college concentration of oxygen exterior the cell and a lower concentration of oxygen inside the prison cell, oxygen molecules lengthened better equally they enter the cell, or the low oxygen concentration side. This is how our red blood cells, low on oxygen, can option up more oxygen in the highly oxygen dumbo lungs.
3. Molecule Accuse or Polarity
The more polar the molecule is, the harder it is to cross through the cell membrane.
The less polar or more nonpolar the molecule is, the easier it is to cross through the cell membrane.
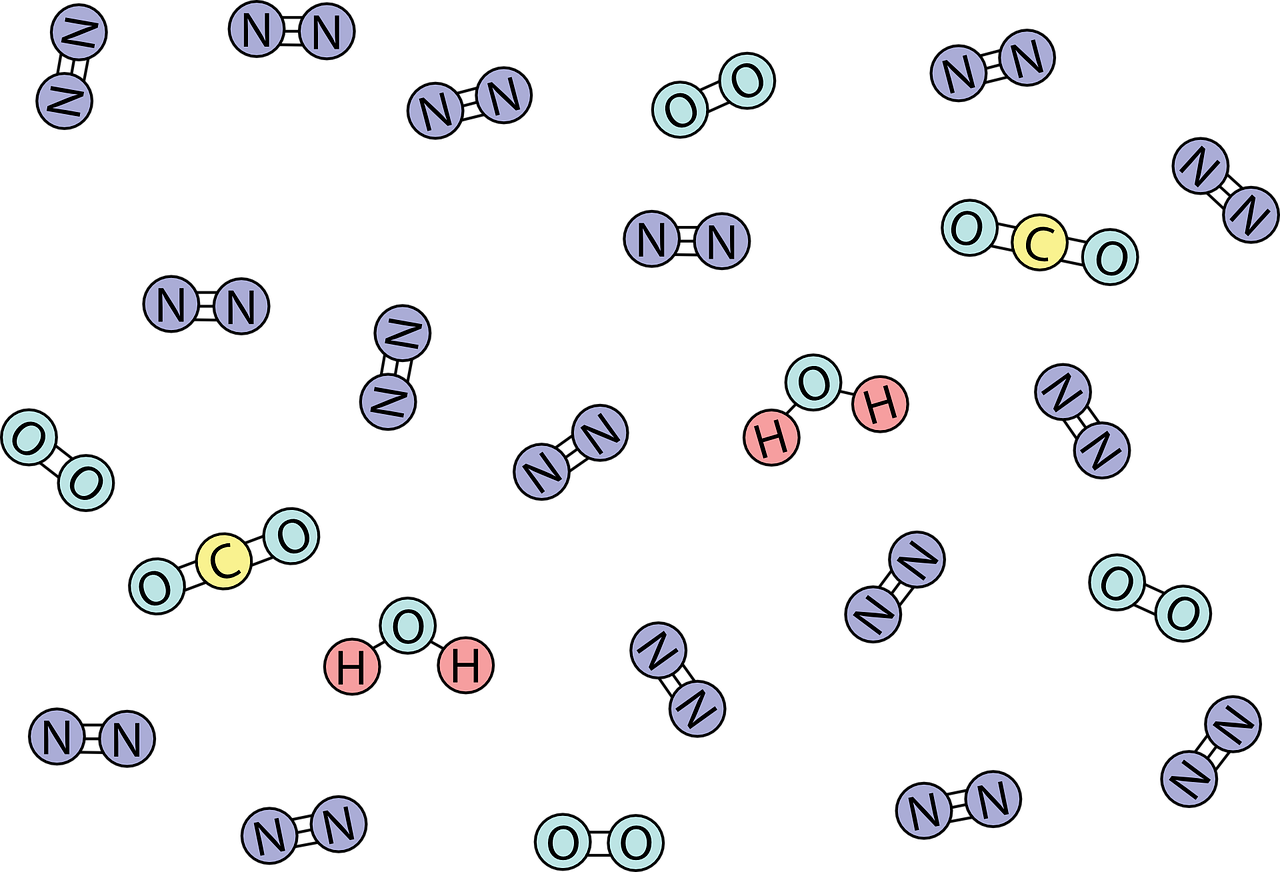
General Order Summary of Molecule Types that can pass through the cell plasma Membrane
All three of these aforementioned factors combine together to play a office on whether or non a molecule or ion can cross through the jail cell membrane, the phospholipid bilayer. In this section, we share a general summary of the types of molecules that tin can diffuse through the cell membrane in society of difficulty of passing through.
Gases such as Oxygen and Carbon Dioxide (CO2) can pass freely through the cell membrane. Pocket-sized polar molecules such as water of H2o tin laissez passer but very slowly. They are usually assisted through facilitated diffusion such as with osmosis.
Large nonpolar molecules such equally benzene are very dull in passing through. The larger the nonpolar molecule, the slower it can laissez passer through the membrane. For example, ethylene is C2H4, which is smaller than the molecular limerick of benzene, C6H12. Since ethylene is smaller than benzene, ethylene can pass through the cell membrane faster relative to benzene (albeit both are slow in passing through compared to gases or modest polar molecules like h2o and ethanol).


The larger the nonpolar molecule, the slower it tin pass through the membrane.
Large polar molecules cannot laissez passer through improvidence. This includes glucose. Lastly, charged polar molecules cannot pass through. Both large polar and charged polar molecules would crave energy or ATP to be transported across the prison cell membrane. This can occur through active transport.
Question: What types of molecules can diffuse easily through a cell membrane?
Putting everything together, pocket-sized nonpolar molecules like oxygen and carbon dioxide tin diffuse easily through a cell membrane. Many ask, "Can h2o lengthened easily through a cell membrane?" Water can diffuse through a cell membrane through aquaporin proteins and osmosis, but water cannot diffuse as easily as pocket-size nonpolar molecules like oxygen and carbon dioxide.
Question: What types of molecules cannot diffuse hands through a cell membrane?
Larger sized and more polar charged molecules cannot lengthened easily through a cell membrane. Examples of molecules that cannot diffuse easily through a cell membrane include glucose and polar charged molecules similar sodium (Na+), potassium (K+), and chloride (Cl-). These molecule types require ATP energy or active transport to laissez passer through the jail cell membrane.
Question: Can ions cross the Lipid Bilayer by unproblematic diffusion?
No, ions cannot cross past simple improvidence or osmosis. Ions are charged molecules. Even if they are pocket-sized sized, their charges create polarity which would not allow them to pass through the lipid bilayer easily. Therefore, ions laissez passer through the cell membrane through active send via poly peptide channels or pumps, or they can cantankerous through the lipid bilayer through facilitated improvidence.
Please annotation that simple diffusion is not facilitated diffusion and that osmosis refers to the movement of water, not ions.
Here is a simplified tabular array summarizing general molecule types that tin pass through the jail cell plasma membrane in order from piece of cake to Difficult.
[Diffuse Easily] Gases (CO2, O2) > Modest Polar (H2O) > Big Nonpolar (Benzene) > Big Polar (Glucose) > Charged Polar Molecules (Cl-, Chiliad+) [Harder to Laissez passer through/Needs Active Transport]
Works Cited
- Nature. Cell Membranes. https://www.nature.com/scitable/topicpage/jail cell-membranes-14052567
- NCBI. The Cell: A Molecular Approach 2nd Edition. https://www.ncbi.nlm.nih.gov/books/NBK9928/
- General Chemistry. 4th Edition. Donald McQuarrie.
© Copyright 2022 Moosmosis – All rights reserved

Back up The states!
Buy usa a loving cup of coffee to support. Our site is run 100% by volunteers from around the world, and nosotros cheers for visiting! Delight donate to support! 🙂
$5.00
Answers to Do Test on "What Types of molecules tin laissez passer through the cell membrane?
- Oxygen can laissez passer through the cell membrane easily because of the nature of its small size! Do non get confused by the catchy reply choices. Oxygen passively crosses the cell membrane and does not need an agile transporter or free energy from ATP.
- *Hint* Red blood cells that just exited out from the lungs are highly oxygenated: high O2 and low Co2. Your trunk cells (legs) have low O2 and high Co2. Think, it'south easier for a molecule to lengthened across the jail cell membrane from a high concentration to a low concentration. Co2 would thus diffuse out from the body cells (high concentration) to the cerise claret cells (low concentration). This does not need whatsoever energy/active send.
- H20! In comparison to the other molecules, H20 is the most polar and thus by relative comparison, cannot pass as easily through the cell membrane. We have to recall, H20 diffuses through the cell membrane with the help of special cell structures called aquaporins. The other 3 answer choices, N2, O2, and Co2 are all nonpolar gasses that pass through the jail cell membrane much easier than H20 can.
Categories: Biology, chemistry
Source: https://moosmosis.org/2019/08/01/cell-membrane-what-types-of-molecules-can-pass-through-the-cell-plasma-membrane/
0 Response to "Molecules That Can Cross the Cell Membrane by Simple Diffusion Are"
إرسال تعليق